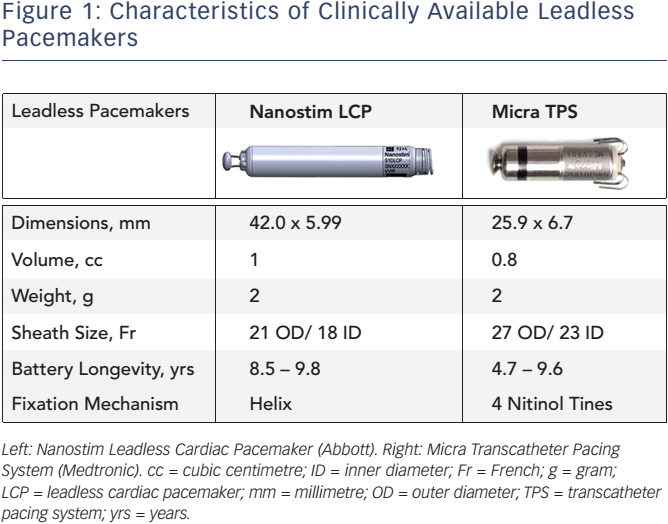
Micra attaches to the right ventricle myocardium via four linear self-expanding nitinol tines. Nanostim which is the size of an AAA battery has no leads and is anchored directly inside one of the hearts chambers.
 Abbott Maintains Nanostim Halt On Docking Button Issue Massdevice
Abbott Maintains Nanostim Halt On Docking Button Issue Massdevice
Do not use the devices with other sheath introducers because this can damage the devices.

Nanostim leadless pacemaker. Jude Medical Nanostim Leadless Pacemaker measures 4 cm long 6 mm wide and 2 g in weight. Jude Medical had begun developing a dual-chamber leadless pacemaker which would be a first if it succeeds. The Nanostim leadless pacemaker and the Micra transcatheter pacing system are implanted directly into the right ventricular apex.
Abbott Park IL is pending approval. Minneapolis MN has been approved by the US Food and Drug Administration for use in the United States. The tiny device is attached directly to the heart eliminating the need for leads.
Although the device was recalled in 2016 owing to rare but serious battery failures the concept of leadless pacing. It is delivered by a catheter threaded up. SELF-CONTAINED LEADLESS PACEMAKERS FOR RIGHT VENTRICULAR PACING Two leadless pacing systems are currently clinically available.
Only one leadless pacemaker Micra Medtronic PLC. The affected device is the Nanostim leadless cardiac pacemaker LCP. In May 2011 Nanostim announced that St.
In May 2011 Nanostim announced that St. 1 the Nanostim Leadless Cardiac Pacemaker. Dont delay your care at Mayo Clinic Schedule your appointment now for safe in-person care.
Abbott Park IL USA was the first self-contained intracardiac pacemaker to be implanted in a human patient. Jude Medical Inc Saint Paul MN USA. Abbott and Medtronic are the only companies that have developed single-chamber leadless pacemakers.
A second Nanostim Abbott Laboratories. A total of 1423 Nanostim devices were implanted worldwide between 2013 and 2016 and three clinical trials were initiated. In May it was announced that the Nanostim leadless pacemaker was successfully retrieved in 14 patients up to 32 years post-implantation without any serious adverse events Though the design and material of Micra enables it to be taken out when needed the intended end-of-life scenario is for it to be left in the body according to Modern Healthcare.
June 4 2018 By Nancy Crotti. As a leadless pacemaker it does not need a connector pacing lead or pulse generator pocket but it has the same operating principles as a conventional pacemaker. Nanostim is an early-stage AIMD company in Milpitas CA that is developing a pacemaker that can be implanted inside the heart through a catheter.
Jude Medical had made a substantial investment in the company. The tiny device is attached directly to the heart eliminating the need for leads. Jude recalled its version Nanostim in October 2017 after receiving 7 reports of lost telemetry and pacing.
The Nanostim leadless pacemaker provides bradycardia pacing as a pulse generator with built-in battery and electrodes for permanent implantation in the right ventricle. Jude Medical had made a substantial investment in the company. Nanostim is an early-stage AIMD company in Milpitas CA that is developing a pacemaker that can be implanted inside the heart through a catheter.
The Nanostim Leadless Pacemaker is intended for use with a Nanostim Programmer Link and a. Nanostim attaches via an active screw-in helix. Even before its acquisition by Abbott St.
The Nanostim Leadless Pacemaker and Nanostim Delivery System Catheter are intended to be used with the Nanostim Introducer Kit. This differs from the. However extraction experience with simultaneous re-implant of a leadless device remains limited.
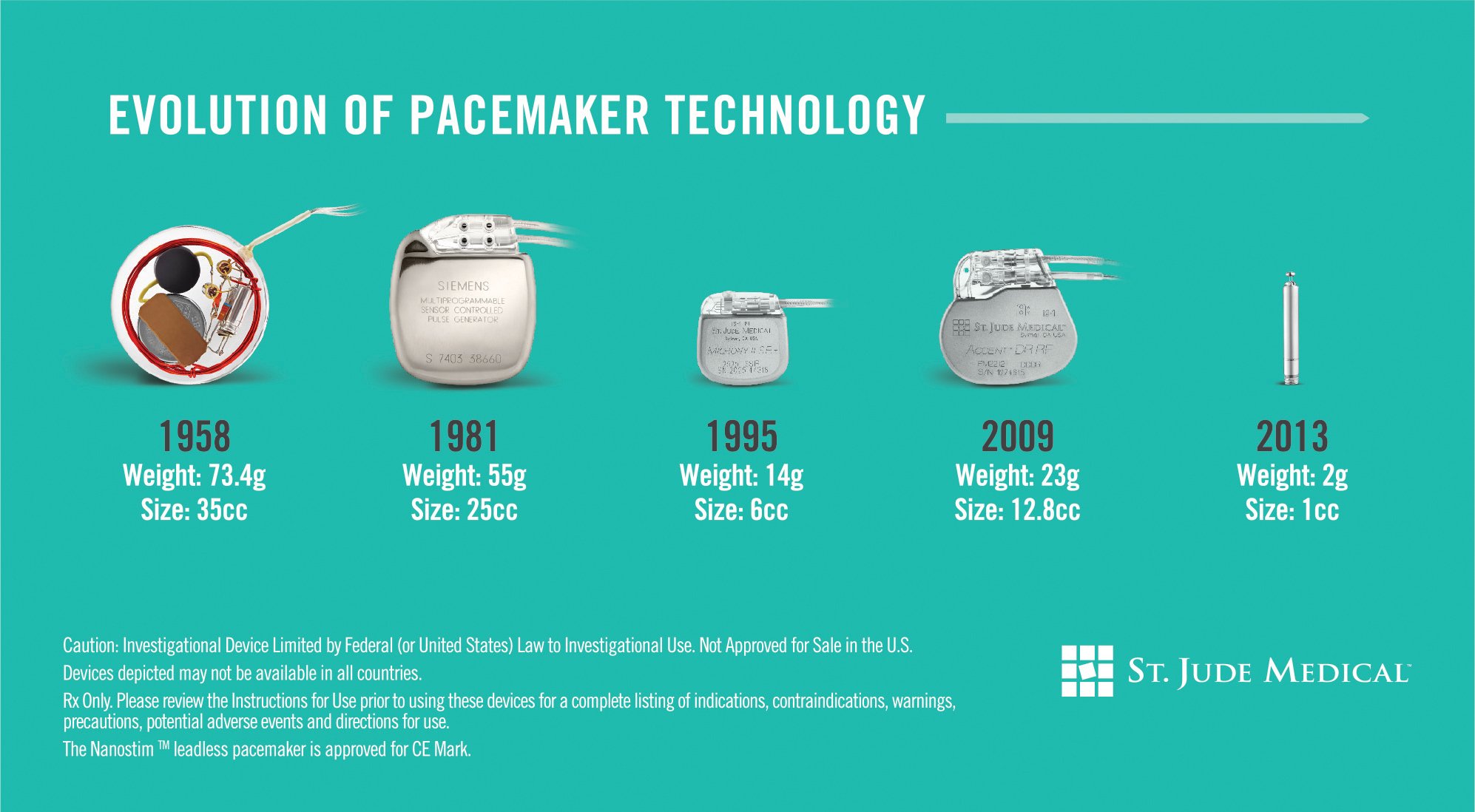
The Nanostim is the worlds first leadless pacemaker to enter the market and is less than 10 percent the size of a conventional pacemaker. Amit Doshi Mercy Clinic electrophysiologist and principal investigator for the NanostimTM LEADLESS II Clinical Trial at Mercy Hospital St. The device is fixated to the right ventricle using a non-retractable helix.
The leadless pacemaker LP is a promising new technology in pacing therapy that avoids a generator pocket and transvenous lead-related complications. Now Abbott Medical Inc. Available leadless pacemaker devices and compare the early results with these devices with historical transve-nous single-chamber pacemaker VVI cohorts.
Https Www Fda Gov Media 95861 Download
 Leadless Pacemakers 14 The Micra Transcatheter Pacing System Left Download Scientific Diagram
Leadless Pacemakers 14 The Micra Transcatheter Pacing System Left Download Scientific Diagram
 Leadless Pacemakers The Size Of A Vitamin Showing Promise Duke Health
Leadless Pacemakers The Size Of A Vitamin Showing Promise Duke Health
Nanostim The World Of Implantable Devices
My Take From Hrs Leadless Pacemakers Are Entering The Mainstream The World Of Implantable Devices
 Nanostim Lcp And Micra Tps The Nanostim Lcp Has A Longer Profile With Download Scientific Diagram
Nanostim Lcp And Micra Tps The Nanostim Lcp Has A Longer Profile With Download Scientific Diagram
 Pacing Supplement Leadless Pacing The British Journal Of Cardiology
Pacing Supplement Leadless Pacing The British Journal Of Cardiology
 The St Jude Medical Nanostim Leadless Pacemaker Reproduced With Download Scientific Diagram
The St Jude Medical Nanostim Leadless Pacemaker Reproduced With Download Scientific Diagram
 End Of Life Management Of Leadless Cardiac Pacemaker Therapy Aer Journal
End Of Life Management Of Leadless Cardiac Pacemaker Therapy Aer Journal
 Nanostim Leadless Pacemaker Gains European Approval Purchased By St Jude Medical Daic
Nanostim Leadless Pacemaker Gains European Approval Purchased By St Jude Medical Daic
 Fda Panel Recommends Deep Long Term Monitoring For Leadless Pacemakers Massdevice
Fda Panel Recommends Deep Long Term Monitoring For Leadless Pacemakers Massdevice
 Safety And Efficacy Of Leadless Pacemaker Retrieval Li 2019 Journal Of Cardiovascular Electrophysiology Wiley Online Library
Safety And Efficacy Of Leadless Pacemaker Retrieval Li 2019 Journal Of Cardiovascular Electrophysiology Wiley Online Library
 Nanostim Leadless Pacemaker Gains European Approval Purchased By St Jude Medical Daic
Nanostim Leadless Pacemaker Gains European Approval Purchased By St Jude Medical Daic
 St Jude Calls Global Stop To Nanostim Implants Over Battery Issues Massdevice
St Jude Calls Global Stop To Nanostim Implants Over Battery Issues Massdevice
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.