Theres one big important difference between Pfizer and Modernas coronavirus vaccines Both Pfizer and Moderna have announced promising vaccine candidates in recent days. A priming dose followed by a booster shot.
 Pfizer Vs Moderna Covid 19 Vaccine What S The Difference Wwlp
Pfizer Vs Moderna Covid 19 Vaccine What S The Difference Wwlp
The Pfizer-BioNTech vaccine was said to be 95 effective.
Difference between pfizer and moderna vaccines. Was granted an EUA by FDA. For the Pfizer vaccine its 21 days. COVID-19 mRNA Vaccines Information about mRNA vaccines generally and COVID-19 vaccines that use this new technology specifically.
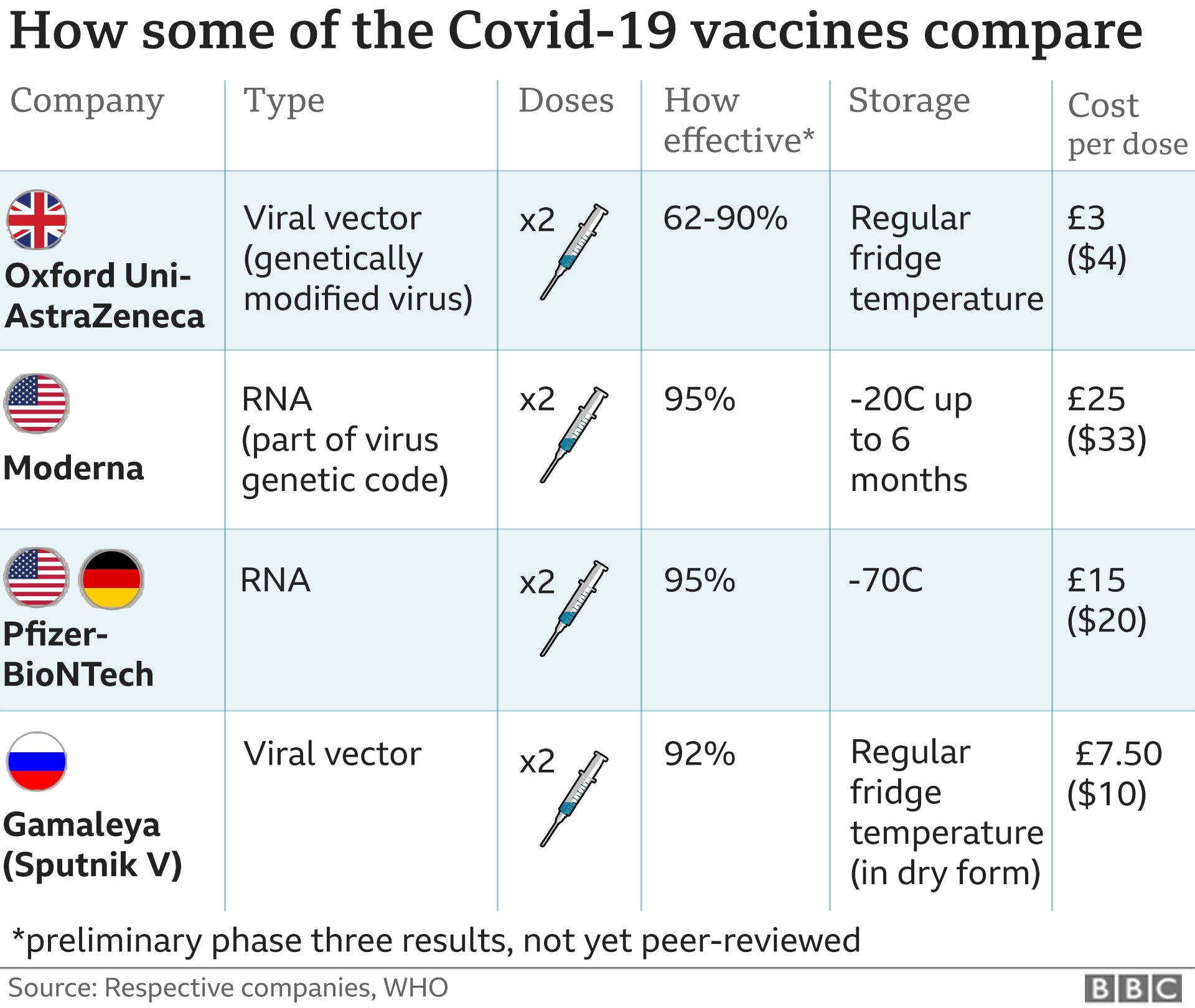
A study of T cell responses shows that mRNA vaccines produced by Moderna and Pfizer-BioNTech are as effective against the UK. Storage distribution and shelf life. Pfizers vaccine is administered as two 30-microgram doses given 21 days apart.
The interval between Moderna doses is 28 days. There are key differences between the COVID-19 vaccines from Moderna and Pfizer. In February 2021 a COVID-19 vaccine developed by Janssen Biotech Inc.
Both the Moderna and the Pfizer vaccines require two shots. Both the Pfizer and Moderna vaccines against COVID-19 require a few weeks wait between the first and second dose. At Moderna this is 18 years or older.
Also see the different types of COVID-19 vaccines that currently are available or are undergoing large-scale Phase 3 clinical trials in the United States. Both need to be stored at freezing temperatures but Pfizers vaccine is -80 to -60 degrees compared to Modernas at -25 to -15 degrees. Storage requirement of between -60 to -80 degrees Celcius ultra-cold freezer.
Pfizers vaccine is approved in our country for anyone aged 16 or older. A comparison of key details for each vaccine can be found below. If authorized the Moderna vaccine would be used in people 18 and older while the Pfizer vaccine was authorized.
They use messenger RNA to induce the body to produce a key protein found on the surface. Until now these vaccines had been only available to the age group 35 with PfizerBioNTech also administered to people aged 16 and 17 while people younger than 35 but older than 18 were supposed to be administered the AstraZeneca vaccines. For further details see the FDA EUA document for PfizerBioNTech Moderna.
A priming dose followed by a booster shot. By PfizerBioNTech and Moderna both vaccines use the same technology mRNA and are highly effective at preventing COVID-19 infection. Differences between Pfizer and Moderna COVID-19 vaccines Lauren Jackson 12122020 Colonial Pipeline restarts after hack but supply.
Each dose of Pfizers contains 30 micrograms of vaccine. A key difference between the two mRNA vaccines Pfizer and Moderna both developed mRNA vaccines for COVID-19. According to Pfizers fact sheet that vaccine specifically is administered intramuscularly injected into the musclecommonly the deltoid in the upper arm in a series of two shots spaced 21.
Both the Moderna and the Pfizer vaccines require two shots. Can be kept in a refrigerator for up to 5 days. The Pfizer vaccine needs to be kept at temperatures around minus 80.
Moderna went with a much larger dose of vaccine 100 micrograms. The primary difference is how they are stored. And South African variants of COVID-19 as they are against the.
The registration website for vaccination now offers the vaccines by Moderna and PfizerBioNTech to all age categories. Covid-19 vaccines and the difference between AstraZeneca Moderna and Pfizer jabs More than 37 million people in the UK have received their first dose of a coronavirus vaccine. A new CDC study reported that a single dose of Pfizers or Modernas COVID vaccine was 80 effective in preventing infections.
This list is not exhaustive. At this temperature it can be stored for up to 6 months. Is one better or worse.
It means the company is using a little more than three times as much vaccine per person as Pfizer. For the Pfizer vaccine its 21 days. The interval between Moderna doses is 28 days.
And the Pfizer vaccine can only remain stable for 5 days. Viral Vector COVID-19 Vaccines. 7 Zeilen As COVID-19 vaccines become more available many are wondering if theyll have the choice between.
Pfizers vaccine must be transported and stored at a temperature of 80 degrees below zero and a thawed bottle can be stored in the refrigerator for 31 days at temperatures of 2 to 8 degrees.