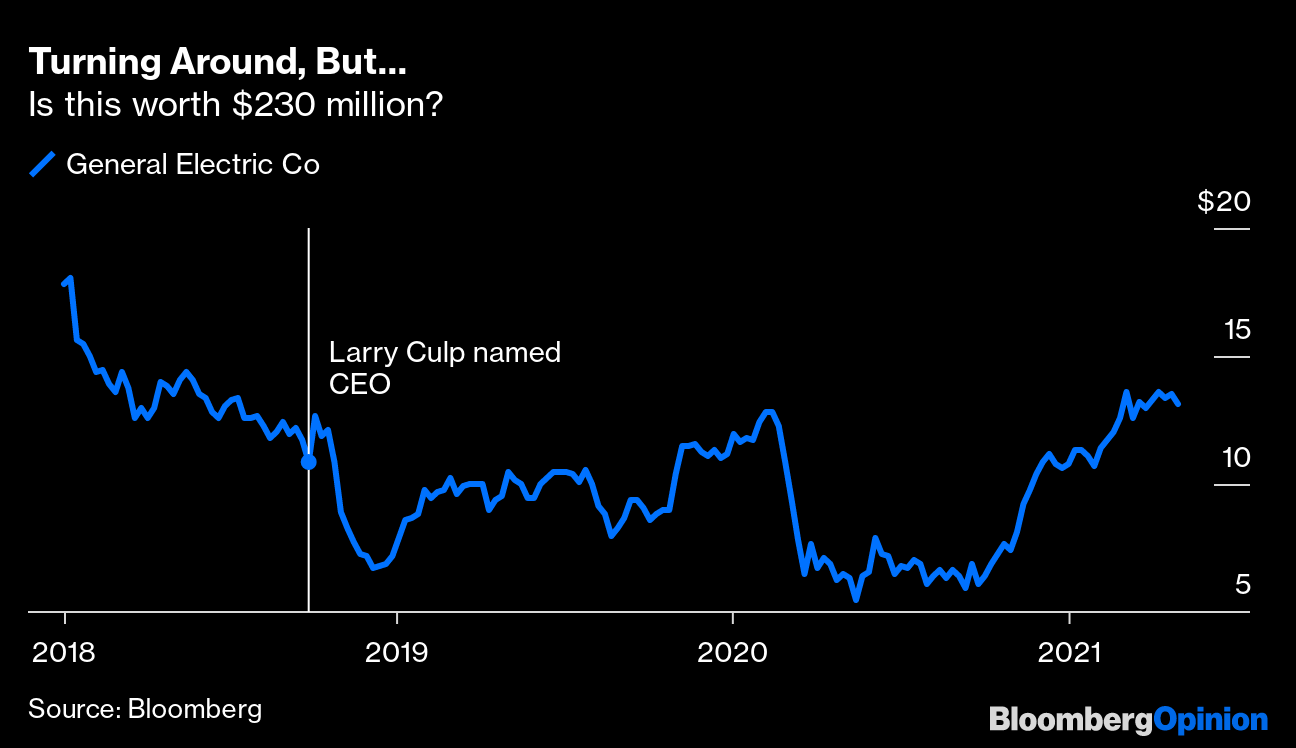
Consensus Panel Chair A Treatment. Regulation 42 CFR 8655 states practitioners with a current waiver to prescribe up to 100 patients and who are not otherwise eligible to treat up to 275 patients under 42 CFR 8610 may request a temporary increase to treat up to 275 patients in order to address emergency situations defined in 42 CFR 82 if the practitioner provides the required information and documentation.
 Why Are So Many Suboxone Patients Buying The Drug On The Street Connecticut Public Radio
Why Are So Many Suboxone Patients Buying The Drug On The Street Connecticut Public Radio
Discharge prescription must be written by X licensed provider.
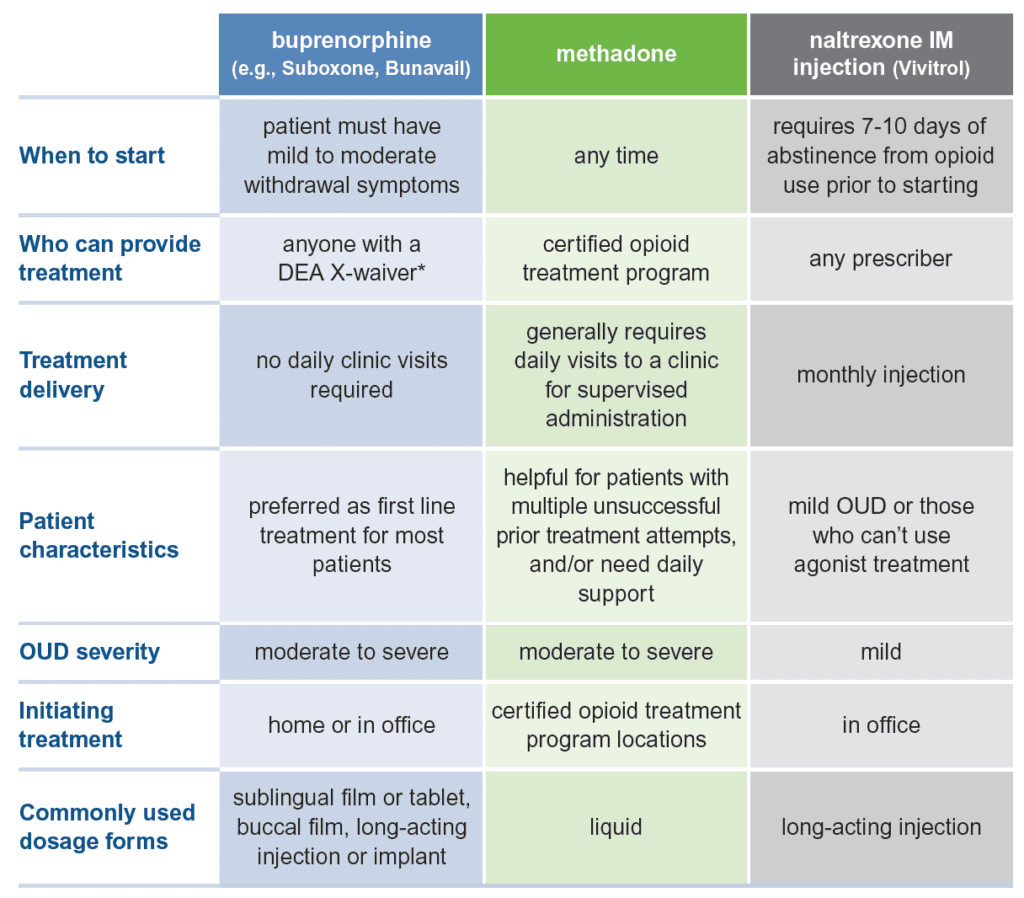
Suboxone prescribing regulations. Prescribers should be contacted for direction after 3 missed doses of buprenorphinenaloxone since this may indicate a considerable loss in patient stability. Every year per 42 CFR 8635 qualified practitioners approved to treat up to 275 patients must submit information about their practice to SAMHSA for purposes of monitoring regulatory compliance. GMC Guidelines for Buprenorphine-Naloxone Suboxone Prescribing Basic Principles Buprenorphine-naloxone is a partial opioid agonist that is approved for treatment of opioid dependence or severe opioid use disorders ICD-10 code.
Buprenorphine-naloxone Suboxone should be dispensed on discharge to discourage diversion. SUBOXONE buprenorphine and naloxone sublingual film for sublingual or buccal use CIII. 812h1 provide that.
1 877 696-2131 To see the latest guidelines research and provincial resources. To expand access to buprenorphine the Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder exempts eligible physicians physician assistants nurse practitioners clinical nurse specialists certified registered nurse anesthetists and certified nurse midwives from the certification requirements related to training counseling and other ancillary. Moderate to severe opioid use disorder Buprenorphine sublingual tablets are considered medically necessary.
A new change in federal rules makes it easier to prescribe a medication that dramatically improves the odds of patients reaching recovery from opioid addiction. Provision of multiple refills is not. SUBOXONE sublingual film is administered sublingually or buccally as a single daily dose.
Exceptions includethe case of pregnancy or breastfeeding. YOU MUST HAVE A SPECIAL X-license in order to prescribe buprenorphine-naloxone. A quick-reference guide for prescribing buprenorphinenaloxone Suboxone in the outpatient setting To speak to an expert in BC.
Prescriber of each missed dose. Rapid Access to Consultative Expertise RACE line. Fter more than 5 doses have been missed the prescription should be.
The SUPPORT Act affords practitioners greater flexibility in the provision of medication-assisted treatment MAT and extends the privilege of prescribing buprenorphine in office-based settings to qualifying other practitioners Nurse Practitioners NPs Physician Assistants PAs Clinical Nurse Specialists CNSs Certified Registered Nurse Anesthetist CRNAs and Certified Nurse. These highlights do not include all the information needed to use SUBOXONE sublingual film safely and effectively. See full prescribing information for SUBOXONE sublingual film.
The goal of the reporting requirement is to ensure that practitioners are providing buprenorphine treatment in compliance with the final rule Medication Assisted Treatment for Opioid. LU codes for Suboxone state that prescribers should complete an accredited course on opioid addic-tion and buprenorphine treatment before prescribing. However in addition it is best practice to also indicate.
Medication should be prescribed in consideration of the frequency of visits. Start and stop. The effect of the three-year provision in DATA 2000 is to put into abeyance current State law or regulations prohibiting physicians from prescribing Subutex or Suboxone for the treatment of opioid addiction and to prevent State regulatory agencies from prohibiting prescribing by regulation.
Medi-Cal covers buprenorphine and buprenorphine-naloxone with some other insurance providers a TAR may be necessary. Suboxone sublingual film Zubsolv sublingual tablet Buprenorphine monotherapy Preferred buprenorphine-naloxone combination products do not require authorization and are considered medically necessary when used for the treatment of. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction Laura McNicholas MD PhD.
The naloxone is not absorbed sublingually and the combination formulations decrease the risk of injectiondiversion. OTPs must ensure that opioid agonist treatment medications are administered or dispensed only by a practitioner licensed under the appropriate State law and registered under the appropriate State and. Other exceptions will be addressed on a case by case basis.
The buprenorphinenaloxone combination product is the preferred formulation at insert. OTP regulations under 42 CFR. The FDA approved Subutex and Suboxone on October 8 2002.
HIGHLIGHTS OF PRESCRIBING INFORMATION. Prescriptions for Suboxone have the same requirements as other straight Narcotic Drugs Schedule N drugs.